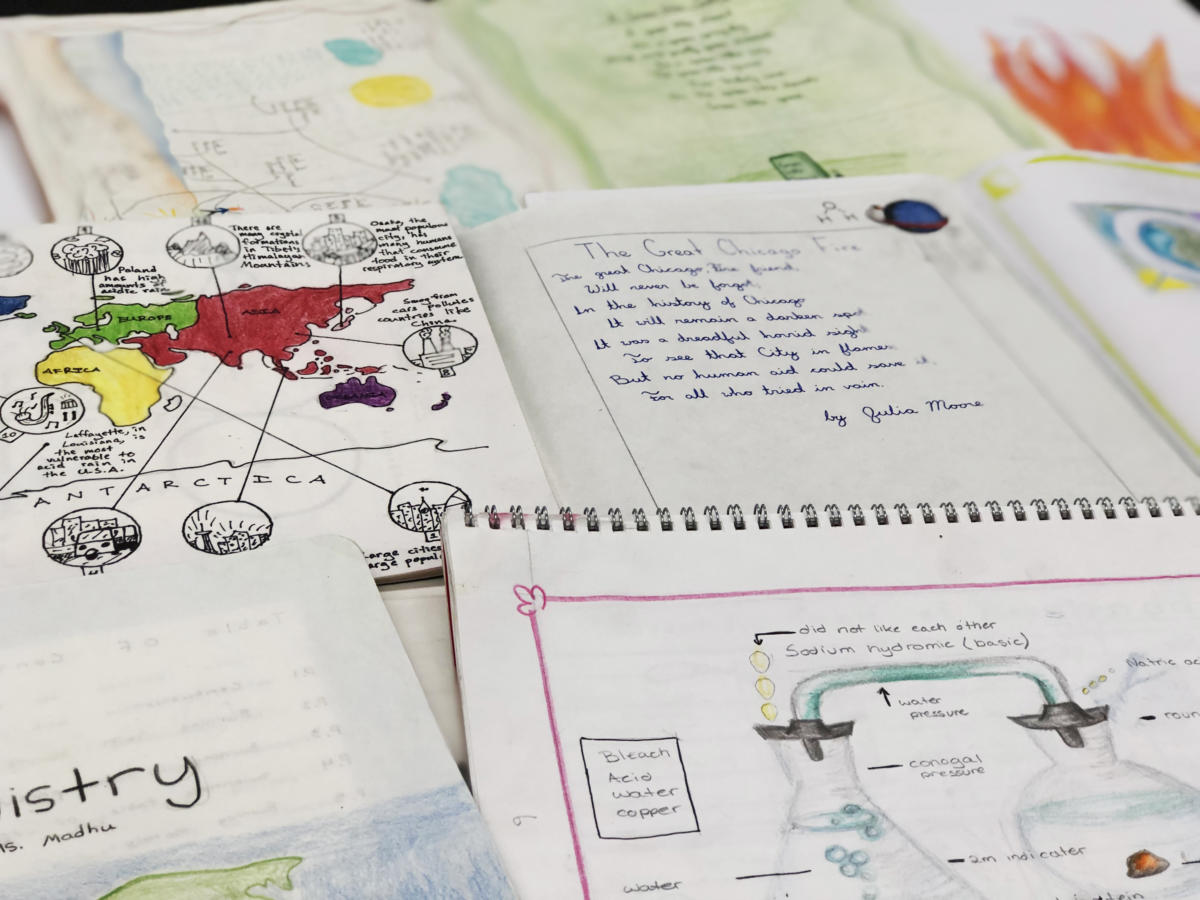
A Chemistry Block in the Seventh Grade
The Chemistry block is designed to open up to the students of the 7th grade the wonders of chemistry.
—Dr. Caroline Martin, Steiner Science Teacher
We take a journey into the nature of matter and use our knowledge to build a connected picture of Earth from the sub-microscopic to the global. We begin by using heat to reveal the forces of energy that hold matter together, allowing the students to connect the cosmic origins of light with everyday objects in their world. By refining the topic further and considering the chemistry of combustion, the students are able to connect the workings of their own bodies (breathing, eating, exercising, etc.) with the transfer of energy that determines the relationships between all objects and chemical elements in our world.
After several displays of energy transfer through burning, we settle into a consideration of water, the life-giving fluid that characterizes Earth. Water brings about chemical change in a more gradual but no less subtle way than burning does, and the students observe a range of experiments dealing with the concepts of density, solubility, acidity, basicity, and saturation.
We consider the pathways that water takes on its cyclical journey through solid Earth and how its chemistry and the chemistry of the local environment comes to be altered along the way. We demonstrate how certain changes to water’s chemistry can render a fluid acidic or basic, giving it the ability to chemically alter matter and thus transfer energy.
The students see how increasing the concentration of dissolved salts in water can lead to a state of saturation, and how the addition of new salts can cause displacement reactions in metals. Taking the concept of saturation to the extreme, we finish the course by reforming matter in the form of crystals. The students learn how changes to the physical environment, such as to temperature and pressure, can determine the extent of crystal growth. In all, we conduct around 13 laboratory experiments covering a wide range of chemical and physical reactions.
The students are assessed on their class and laboratory participation, class quizzes, and their Main Lesson Book pages. They also complete a final project, which takes the form of a field trip to Central Park in an endeavor to identify each of the several experiments in the real world. The fruits of this treasure hunt are illustrated and presented by the students in the form of a Main Lesson Book page.